Abstract
Background Axicabtagene ciloleucel (axi-cel) is an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved for patients (pts) with large B-cell lymphoma (LBCL) refractory to or relapsed within 12 months of first-line chemoimmunotherapy and pts with relapsed or refractory (R/R) LBCL after ≥2 lines of prior systemic therapy. Following axi-cel infusion, Grade ≥3 neurologic events (NEs) and cytokine release syndrome (CRS) were reported in the ZUMA-1 registrational study (NE, 32% of pts; CRS, 11%) and Phase 3 ZUMA-7 study (NE, 21%; CRS, 6%) (Locke. Lancet Oncol. 2019; Locke. NEJM. 2021). Granulocyte-macrophage colony-stimulating factor (GM-CSF) levels after infusion were positively associated with Grade ≥3 NEs in ZUMA-1 (Neelapu. NEJM. 2017) and both Grade ≥3 NEs and CRS in ZUMA-7 (Filosto. AACR #CT004. 2022). Lenzilumab is a humanized anti-GM-CSF IgG1κ monoclonal antibody. Preclinical evidence indicates that inhibition of the GM-CSF axis with lenzilumab may suppress CAR T-cell toxicity without impairing efficacy (Sterner. Blood. 2019). Here we report Phase 1 results of ZUMA-19 (NCT04314843), a single-arm study evaluating the safety of axi-cel in combination with lenzilumab in adult pts with R/R LBCL.
Methods Eligible pts were ≥18 years old, with chemorefractory LBCL or relapsed LBCL after ≥2 prior lines of therapy, and ECOG PS 0-1. Phase 1 of ZUMA-19 comprised 2 lenzilumab dose-finding cohorts of pts who received single infusions of lenzilumab 600 mg intravenous (IV) (Cohort 1) or 1,800 mg IV (Cohort 2) 6 hours prior to receiving axi-cel. All pts underwent lymphodepletion with cyclophosphamide 500 mg/m2/day and fludarabine 30 mg/m2/day before a single infusion of axi-cel at a target dose of 2 × 106 cells/kg. Primary endpoint was incidence of dose-limiting toxicities (DLTs). Key secondary endpoints were safety (including NEs and CRS), efficacy, lenzilumab and CAR T-cell pharmacokinetics, and cytokine levels in the blood. CRS was graded according to a revised system per Lee et al (Blood. 2014). Other adverse events (AEs) including NEs were graded according to National Cancer Institute Common Terminology Criteria for AEs (v4.03).
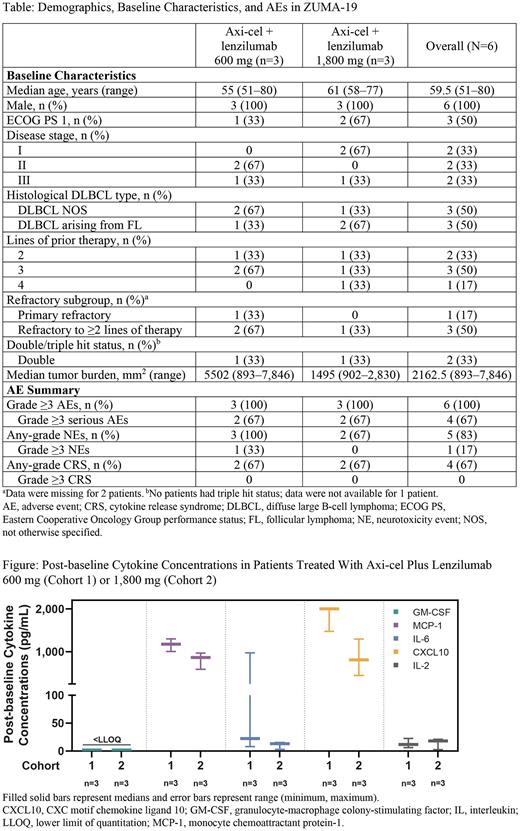
Results At the data cutoff date (May 9, 2022), 6 pts had received treatment with axi-cel plus lenzilumab in Cohort 1 (n=3) and Cohort 2 (n=3). Overall, median follow-up was 17.1 months (range, 14.7-22.3 months). Median age was 59.5 years (range, 51-80 years), 50% of pts had an ECOG PS of 1, and 50% of pts were refractory to ≥2 lines of therapy (Table). Median tumor burden was 2,162.5 mm2 (range, 893-7,846 mm2). No DLTs or Grade ≥3 CRS events were observed in either cohort. A single Grade ≥3 axi-cel-related NE (encephalopathy) was observed in Cohort 1; no Grade ≥3 NEs were observed in Cohort 2. Median onset of worst-grade NE in Cohort 1 and Cohort 2 was 8 and 9.5 days, respectively. No lenzilumab-related AEs occurred in either cohort. Four of 6 pts died (67%; COVID-19 pneumonia, n=3; refractory disease, n=1); no deaths were treatment related. Objective and complete response rates were 83% and 67%, respectively. Of 5 pts with a response, 4 had a response within 30 days; none had subsequent disease progression. Lenzilumab serum concentrations were dose proportional, with Cmax values of 67.1-89.9 µg/mL in Cohort 1 and 249-299 µg/mL in Cohort 2. Lenzilumab was sustained above a target serum concentration of 50 µg/mL through Day 1 in Cohort 1 and Day 4 in Cohort 2. Peak concentrations of circulating CAR T cells (normalized to baseline tumor burden) were comparable between cohorts (range, 0.001-0.03 cells/µL*mm2 in both cohorts). Peak CAR T-cell concentrations occurred between Days 7 and 14 after infusion. Post-baseline GM-CSF levels were below the lower limit of quantitation in all pts at all time points through at least Week 4. Across Cohorts 1 and 2, there were lenzilumab dose-dependent reductions in serum concentrations of proinflammatory myeloid-related cytokines and chemokines (MCP-1, IL-6, IL-8, CXCL10, TNFα, and IL-1RA) and IFNγ compared with no reduction in IL-2 (Figure). Abundance of circulating intermediate monocytes and the acute phase proinflammatory marker ferritin were also lower in Cohort 2 compared with Cohort 1.
Conclusions No new safety signals or DLTs were observed in pts treated with axi-cel plus lenzilumab. Addition of lenzilumab to the axi-cel regimen appeared to dose-dependently suppress the GM-CSF axis and reduce markers of systemic inflammation.
Disclosures
Oluwole:ADC Therapeutics: Consultancy; Kite, a Gilead Company: Research Funding; Curio Science: Consultancy; TG Therapeutics: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Pfizer: Consultancy. Kenderian:Mettaforge: Patents & Royalties; MustangBio: Patents & Royalties; Life Engine: Current holder of stock options in a privately-held company; Morphosys: Research Funding; LEAH Labs: Current holder of stock options in a privately-held company, Research Funding; Viracta/Sunesis: Research Funding; Tolero: Research Funding; Lentigen: Research Funding; Humanigen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: CART cell therapy , Research Funding, Speakers Bureau; Juno/BMS: Consultancy, Research Funding, Speakers Bureau; Kite/Gilead: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Patents & Royalties: CART cell therapy , Research Funding, Speakers Bureau. Shiraz:Kite, a Gilead company: Research Funding. Karmali:AstraZeneca: Other: Advisory Board, Speakers Bureau; Kite: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau; Takeda: Research Funding; Calithera: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; Morphosys/Incyte: Consultancy, Other: Advisory Board, Speakers Bureau; Genentech/Roche: Consultancy, Other: Advisory Board; Pharmacyclics: Consultancy, Other: Advisory Board; BMS/Celgene: Consultancy, Research Funding; Karyopharm: Consultancy; Eusa: Consultancy; BeiGene: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau. Reshef:Gilead: Consultancy, Honoraria, Other: Travel Support, Research Funding; Novartis: Honoraria; Atara Biotherapeutics: Consultancy, Research Funding; Jasper: Consultancy; MidaTech: Consultancy; Regeneron: Consultancy; Synthekine: Consultancy; TScan: Consultancy; Bayer: Consultancy; Incyte: Research Funding; Pharmacyclics: Research Funding; Shire: Research Funding; Immatics: Research Funding; Precision Biosciences: Research Funding; Takeda: Research Funding; J&J: Research Funding; BMS: Honoraria; Capstan Therapeutics: Consultancy; University Of Pennsylvania: Other: Data Safety Monitoring Board. McCarthy:Takeda Pharmaceuticals America, Inc.: Consultancy, Honoraria; Fate Therapeutics: Consultancy, Honoraria; Starton Therapeutics: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Sanofi: Consultancy; Magenta Therapeutics: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Oncopeptides: Consultancy, Honoraria; Partner Therapeutics, Inc.: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bluebird Bio: Consultancy, Honoraria; Janssen Global Services, LLC: Consultancy, Honoraria; Karyopharm Therapeutics Inc.: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Juno: Consultancy, Honoraria; Axios: Consultancy, Honoraria; Bristol Myers Squibb Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ghosh:Janssen: Consultancy, Speakers Bureau; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Gilead: Consultancy, Research Funding, Speakers Bureau; Beigene: Consultancy; Incyte: Consultancy; Karyopharm: Consultancy; Roche/Genentech: Consultancy, Research Funding; Novartis: Consultancy; Loxo Oncology: Consultancy; Genmab: Consultancy; Adaptive Biotech: Consultancy; ADC Therapeautics: Consultancy; Epizyme: Speakers Bureau; Morphosys: Research Funding; AbbVie: Research Funding; Pharmacyclics: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; TG Therapeautics: Consultancy, Research Funding; Seagen: Consultancy; Levine Cancer Institute: Current Employment, Honoraria, Research Funding. Lazaryan:Sanofi: Consultancy; Humanigen: Consultancy; Teladoc: Current equity holder in publicly-traded company; AmWel: Current equity holder in publicly-traded company; AvroBio: Consultancy. Filosto:Tusk Therapeutics: Patents & Royalties; Kite, a Gilead Company: Current Employment, Current equity holder in publicly-traded company. Poddar:Kite, a Gilead Company: Current Employment, Current equity holder in publicly-traded company; UCLA: Other: intellectual Property, Patents & Royalties. Peng:Kite, a Gilead Company: Current Employment; Gilead Sciences: Current equity holder in publicly-traded company. Kilcoyne:Humanigen: Current Employment, Current holder of stock options in a privately-held company. Nahas:Kite, a Gilead company: Current Employment, Current holder of stock options in a privately-held company. Neelapu:Bluebird Bio: Consultancy, Honoraria; Unum Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Calibr: Consultancy, Honoraria, Other: Personal fees; Adicet Bio: Consultancy, Honoraria, Other: Personal fees, Research Funding; Legend Biotech: Consultancy, Honoraria, Other: Personal fees; Precision Biosciences: Consultancy, Honoraria, Other: Personal fees, Research Funding; Incyte: Consultancy, Honoraria, Other: Personal fees; Cell Medica/Kuur: Consultancy, Honoraria, Other: Personal fees; Allogene Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Pfizer: Consultancy, Honoraria, Other: Personal fees; Celgene: Consultancy, Honoraria, Other: Personal fees, Research Funding; Novartis: Consultancy, Honoraria, Other: Personal fees; Bristol Myers Squibb: Consultancy, Honoraria, Other: Personal fees, Research Funding; Merck: Consultancy, Honoraria, Other: Personal fees, Research Funding; Kite: Consultancy, Honoraria, Other: Personal fees, Research Funding; Medscape: Consultancy, Honoraria; Aptitude Health: Consultancy, Research Funding; Bio Ascend: Consultancy, Honoraria; Poseida: Research Funding; Cellectis: Research Funding; Karus Therapeutics: Research Funding; Acerta: Research Funding; Takeda Pharmaceuticals: Patents & Royalties: related to cell therapy..
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal